Introduction
The Mediterranean Sea is the world’s tenth-largest sea, spanning over 2.5 million km2. It is made up of 12 smaller seas, including the Aegean Sea, which is situated between Greece and Turkey. The Aegean Sea has a surface area of 215,000 km2 and a maximum depth of 3,544 m (World Atlas, 2021).
The striped dolphin (Stenella coeruleoalba) is a pelagic small delphinid common in warm-temperate to tropical waters globally (Archer, 2018). They are easily identifiable by their distinctive pattern of blue or bold grey and white stripes and blazes along the lateral and dorsal sides of their bodies (Archer, 2018; Figure 1). They are not considered an endangered species but are listed as “Vulnerable” on the IUCN red list (Gaspari et al., 2007; Cozzi et al., 2017). S. coeruleoalba often travels in large groups of 25 to 100 individuals; they feed on small pelagic fish and squid and can live up to 55-60 years (Cozzi et al., 2017; Society for Marine Mammalogy, 2022).
Dolphins, like many mammal species, live in fission–fusion societies where individuals join and leave groups on a flexible basis, with group composition and size changing frequently on small spatial and temporal scales (Blasi and Boitani, 2014). High rates of group composition changes in fission–fusion societies are thought to be an adaptive response to the dynamic interaction of ecological variables (Blasi and Boitani, 2014). Several factors potentially affect the social structure and subgroups of these dolphins, including habitat structure (depth, distribution), predation risk, prey distribution, cultural transmission, and male competition (Mesnick et al., 2019).
This report aims to analyse the effect of environmental factors on the social structure of Stenella coeruleoalba in the Eastern Aegean Sea. The Aegean Sea contains a trench north-west of Samos Island, in the Icarian Sea, which has a maximum depth of 1,262 m (World Atlas, 2021). This provides a broad depth range across which the species can be observed and allows for the investigation of partitioning sub-groups by depth.

Figure 1. An image of a striped dolphin, Stenella coeruleoalba (Tethys Research Institute, 2020).
The main objectives of this study were to increase the knowledge of the social structure of a Stenella coeruleoalba subpopulation sighted from September 2017 until December 2022 in the eastern Aegean Sea and to analyse whether environmental factors affect their subgroups, as there is currently a lack of information on this topic. The study was conducted by collecting data on the S. coeruleoalba subpopulation by photo identification and recording how often individuals were seen together. The development of understanding of this topic is essential to help establish marine protected areas (MPAs) and for the future conservation of the striped dolphin, such as maintaining their genetic diversity (Gaspari et al., 2007).
The null hypotheses include that there are no distinguishable S. coeruleoalba social subgroups in the eastern Aegean Sea, the social subgroups of S. coeruleoalba will not vary in size, and that S. coeruleoalba subgroups are not defined by geographic location, depth, and distance from shore. The alternative hypotheses include that there are multiple S. coeruleoalba social subgroups in the eastern Aegean Sea, the S. coeruleoalba social subgroups differ in size, S. coeruleoalba social subgroups are fluid, some individuals are seen in multiple subgroups at different times, and that S. coeruleoalba subgroups can be defined by geographic location, depth, and distance from shore.
Materials and Methods
Study Area
The study area was the Aegean Sea around the Northeast Aegean Islands including along a trench on the north-western side of Samos Island and north of Ikaria Island. (Figure 2a). Boat surveys were carried out from September 2017 until December 2022 over the whole year. The total area of the trench is 8013,637 km2. The depth varies between 25 m along the edge and 1250 m at its deepest (Figure 2b). The starting point for each survey was various ports on the nearby islands. These were the Archipelagos Marine Life Sanctuary or port of Lipsi, the port Marina of Pythagorio, Marathokampos, Karlovassi and Vathy on Samos and the port of Ikaria.
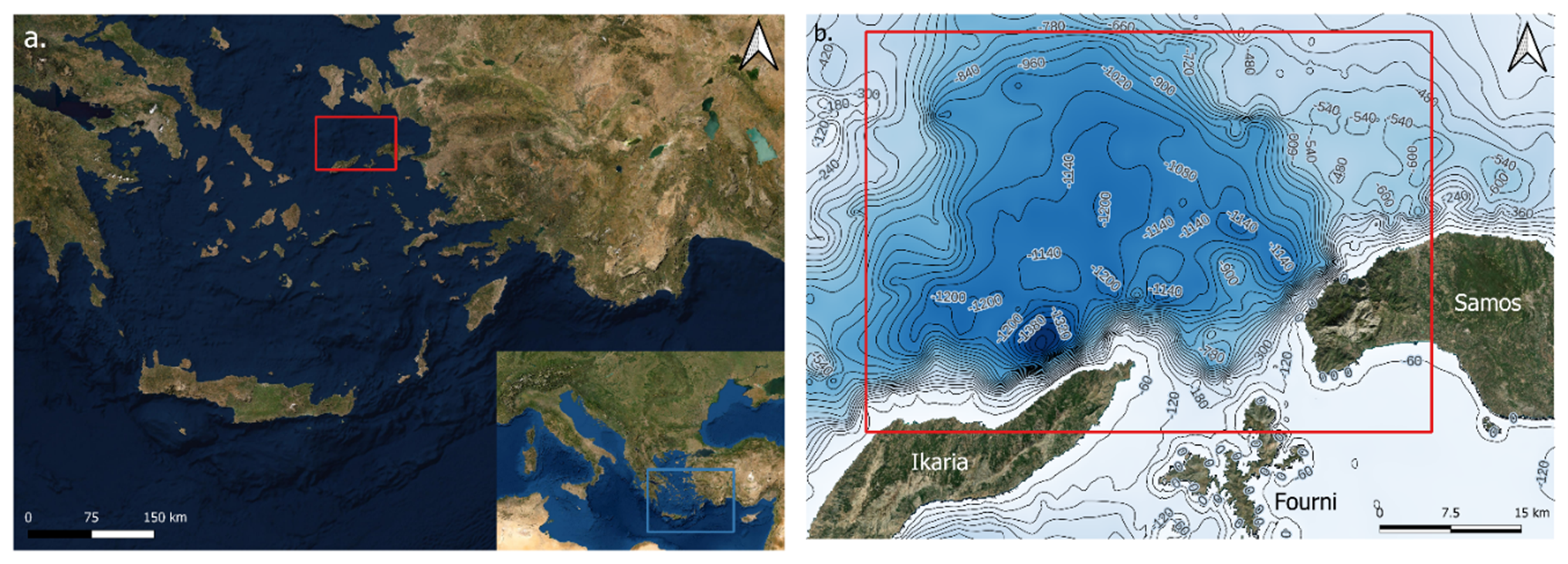
Figure 2. (a) A map of the location of the study area in the Mediterranean Sea shown by the blue box and the location of the study area in the Aegean Sea shown by the red box. (b) Bathymetry map of the trench, the red box showing the study area. Depth shown in metres. Maps plotted using QGIS.
Resources and Equipment
Boat surveys were conducted on the Archipelagos Institute’s research vessels: the Aegean Explorer, Pinelopi, Okeanos and Naftilos. The boat survey crew consisted of six to ten people, including a captain, a supervisor, and multiple observers. Boat surveys were carried out only in good weather conditions of low wind and good visibility (Beaufort Sea State ≤ 3, Douglas Scale ≤ 3) and only during daylight hours, for the crew’s safety and because sightings are harder to identify in harsher conditions and low light. Also, environmental conditions had to be ideal and consistent for high-quality photos. Two photographic digital single-lens reflex cameras, a Nikon D3100 and a Canon EOS 1300D were used to collect identification photographs of each S. coeruleoalba individual focusing on the dorsal fin.
Field Methods and Data Collection
Transects were used to survey the study area. Their duration was most commonly full day, from sunrise to sunset, however, they varied based on weather conditions and the area surveyed. Transects ranged from straight lines to U-shaped curves and searching was conducted as far as the eye could see either side of the boat. The boat maintained a speed of approximately 6.5 knots, during surveys, to minimise the disturbance to the dolphins.
Dolphins are spotted by a continuous scanning method of the sea surface from all angles by four to five trained observers, using either binoculars or the naked eye, by sighting cues (splash, dive, boat, birds) on a predetermined transect for the day. Observers rotated their positions every 30 minutes of survey effort.
When a group of Stenella coeruleoalba was sighted, it was followed until all the individuals had been photographed, it was lost from view or the reaction to the research vessel was negative. Photographs of the dorsal fin of each individual were taken. The group membership (ID photographs), number of individuals, relative cohesion/dispersion and general behavioural states were recorded as well as geographical position for movement patterns. Dolphins were differentiated from incidental events that have left scars or nicks or if they are completely unmarked. An in-depth field methods and data collection methodology description can be found in the supplementary material.
Photos of S. coeruleoalba individuals were taken perpendicular to the dolphin’s body axis concentrated on the dorsal fin, at a 90° angle. The posterior edge (trailing edge) of the dorsal fin is the most obvious feature that is unique for each dolphin. Burst and sports mode with specialised tracking autofocus camera settings were used to take multiple pictures of the surfacing dolphin (at approx. 8 fps). This allowed for a higher probability of capturing the best moments for focused quality pictures (dorsal fins completely out of the water and no/minimum number of splashes). Pictures of as many individuals of the group as possible were taken and both sides of the boat were covered with the two cameras so that pictures of both sides of the dolphin’s dorsal fins could be acquired. Focus was placed on specific individuals in closer proximity or better lighting, until good pictures of them were acquired, before moving on to the next individual.
Photo-ID Analysis
Once back from a survey, photo identification analysis was done by following Brown & Tintore’s protocol (Brown and Tintore, 2021). Only images suitable for photo-ID were used, which was based on an evaluation of the focus/clarity, contrast, and angle of each image (Figures 3,4,5, and 6). The software XnView MP was used as a photo viewer and image resizer for internal and external photo ID matching. Photo identification analysis was done by following Brown and Tintore’s protocol, to keep the database in order (Brown and Tintore, 2021). All photos were uploaded onto a Photo-ID hard drive. Images suitable for photo-ID, that were clear and distinctive, were placed into a sub-folder and any photos that were out of focus or had bad lighting were discarded. Photos were also used to estimate group size. If the dorsal fin of an individual was not identifiable, but permanent markings on the body could be used, the photos were kept. The software XnView MP was used as a photo viewer and image resizer. The images were cropped, so just the fin and body were kept in the image.
In XnView MP, internal matching was completed, where photos from a single group were compared for duplicates of the same individual. It was conducted by opening two windows, one with the image of the fin to match and one window to click through the rest of the pictures from the encounter. Once all internal matching was done, external matching was completed in the same way using the photo-ID catalogues where photos from other groups were compared for duplicates of the same individual. The chosen identifying catalogue image for each individual were chosen depending on whether the fin that was being matched was well marked, slightly marked or only identifiable from one side of the dorsal fin (left or right marked). The individuals were matched to catalogue images by comparing the new images to those in the photo-ID catalogue until a match was found. Then, the image was renamed with the individual name/code. If there were unidentified individuals, they were checked by another observer to ensure that it was a new individual, and it was added to the catalogue.
The picture quality was visually assessed based on an evaluation of the focus/clarity, contrast, and angle of each image. Distinctiveness was related to how distinctive the markings on the individual’s fin were, and how easily the individual could be identified in varying levels of picture quality. ‘Very distinctive’ individuals had unique major nicks/notches on both sides of the dorsal fin. ‘Medium’ distinctiveness individuals had fewer distinguishable markings or minor nicks/notches but may have had a distinguishable pigmentation pattern. ‘Non-distinctive’ individuals had no distinctive nicks/notches, nor a distinguishable pigmentation pattern (Figures 3,4,5, and 6).

Figure 3. Images showing excellent focus, moderate focus, and poor focus (Brown and Tintore, 2021).

Figure 4. Images showing ideal contrast and excessive/minimal contrast (Brown and Tintore, 2021).

Figure 5. Images taken from 90° angle (perpendicular), slight angle, and oblique angle (Brown and Tintore, 2021).

Figure 6. Images the distinctiveness of markings: highly distinctive and non-distinctive (Brown and Tintore, 2021).
Social Structure Analysis
To analyse the social structure, the photo-ID catalogue was used to find all recorded individuals of S. coeruleoalba that have been sighted three or more times. Using the SOCPROG software program, the half-weight association index (HWI) was used to describe cetacean associations, because it displays less bias when not all associates are identified (Whitehead, 2009). The HWI between two individuals (a and b) was calculated by SOCPROG using the following formula:
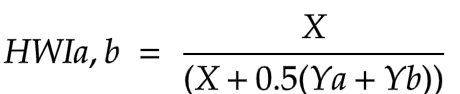
Where X is the number of sightings during which individuals a and b were seen together, Ya is the number of sightings when a was seen without b, and Yb is the number of sightings when b was seen without a (Barendse et al., 2013). The association matrix (HWI values, rounded to the nearest 0.1) generated by SOCPROG was then visualised and analysed by creating a social network diagram and a circular analysis graph.
Data Analysis
QGIS was used to calculate the distance from shore for each individual, using the NNJoin Plugin.
SOCPROG was used to find the half-weight association index (HWI) and the association matrix (HWI values) to further explore the distribution and social structure of S. coeruleoalba. Social network diagram figures were outputted by SOCPROG. R Studio was used to create boxplots showing the difference in depth and distance from shore for each subgroup (RStudio Team, 2020). It was also used to test depth and distance from shore for normality using a Shapiro-Wilk test (Shapiro and Wilk, 1965). As all the data was normally distributed, a one-way ANOVA was performed to test for a significant difference between subgroups. As there was no significant difference, a post-hoc test was not performed.
Results
A total of 27 individuals of Stenella coeruleoalba, sighted more than three times between September 2017 and December 2022 in the north-eastern Aegean Sea, were identified and included in this study. The results of the study support the hypotheses that there are multiple S. coeruleoalba social subgroups in the eastern Aegean Sea and that the social subgroups differ in size. The results also conclude that S. coeruleoalba social subgroups are fluid because some individuals are seen in multiple subgroups. However, the results do not support the hypotheses that the S. coeruleoalba subgroups are defined by geographic location, depth, or distance from shore.
Social Structure
Social Network Diagram
The association matric values indicate three distinct subgroups and one individual without connections to the identified subgroups in this subpopulation. Individual 044 had no social association with any of the individuals included in this study. However, there were individuals that could not be identified in this study, which had social associations with Individual 044. There are three individuals recorded in subgroup A, 12 individuals in subgroup B and nine individuals in subgroup C. The results, therefore, support the hypothesis that there are multiple S. coeruleoalba social subgroups in the eastern Aegean Sea. The study also supports the identification of the largest social subgroup of the population, consisting of 12 individuals. It can also be seen in the social network diagram, that subgroup B had a higher number of connections with the other subgroups, in comparison to the connections between subgroup A and C. Additionally, the results support the alternative hypothesis that S. coeruleoalba social subgroups are fluid and that some individuals are seen in multiple subgroups. The half-weight index lines connect individuals of different subgroups together. The association was strong between individuals in different subgroups showing that there have been sightings of individuals from different subgroups together (Figure 7).
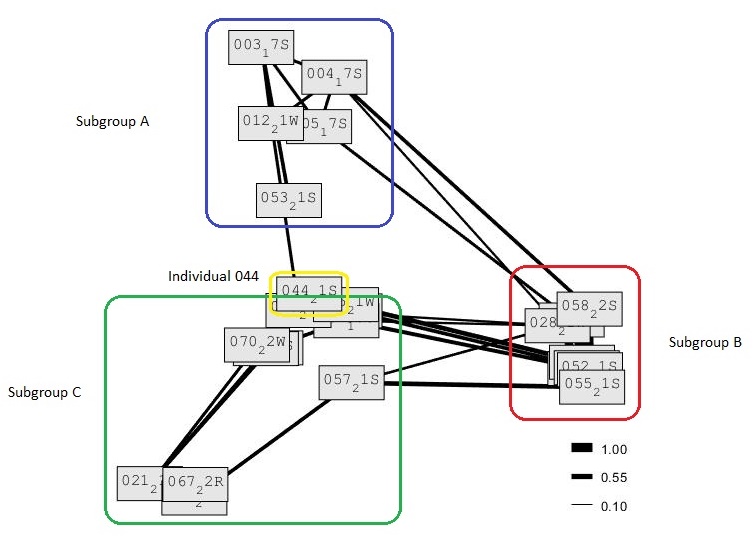
Figure 7. Social network diagram showing the social structure and the association matrix of Stenella coeruleoabla, with the Half Weight Index values rounded to the nearest 0.1. The closer to 1 the value is, the higher the half weight index and the stronger the association between individuals. Graph created using SOCPROG.
Circular Analysis Graph
The circular analysis graph lends support to the hypothesis that S. coeruleoalba social subgroups are fluid and that some individuals are seen in multiple subgroups. The thicker the half weight index line between individuals, the stronger the association between the two. Individual 044 had a half weight index value of 0, as it has no lines attached to it, indicating it has no associations with any other individuals or a particular subgroup included in this study. All other individuals have at least two lines connecting them to other individuals and therefore, to a subgroup. Individual 066 and individual 067 were sighted three times, and on all occasions, they were sighted together. Their half weight index value is 0.8, showing that they have a strong association. Individual 013 and individual 032 are from different subgroups, however, they have a half weight index value of 0.55, suggesting an association. In addition, Individual 028, from subgroup B, had associations with two individuals from the other subgroups, supporting the hypothesis that S. coeruleoalba social subgroups are fluid and that some individuals are seen in multiple subgroups (Figure 8).
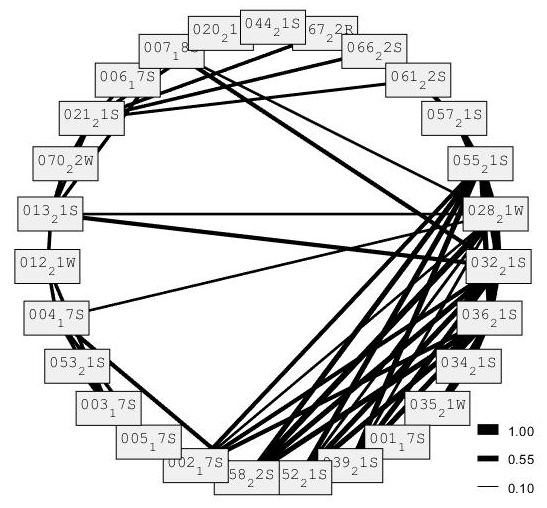
Figure 8. Circular analysis graph showing the association matrix of Stenella coeruleoabla, with the Half Weight Index values rounded to the nearest 0.1. The closer to 1 the value is, the higher the half weight index and the stronger the association between individuals. Graph created using SOCPROG.
Effect of Environmental Factors on Social Structure
Distribution Map
There was a wide distribution of Stenella coeruleoalba sightings mainly along the trench area transects, at a wide range of depths. All subgroups were evenly distributed along the trench with no subgroup dominating a distinct area. The results suggest that S. coeruleoalba subgroups are not defined by geographic location as there were no clearly defined areas where a subgroup was most sighted (Figure 9).
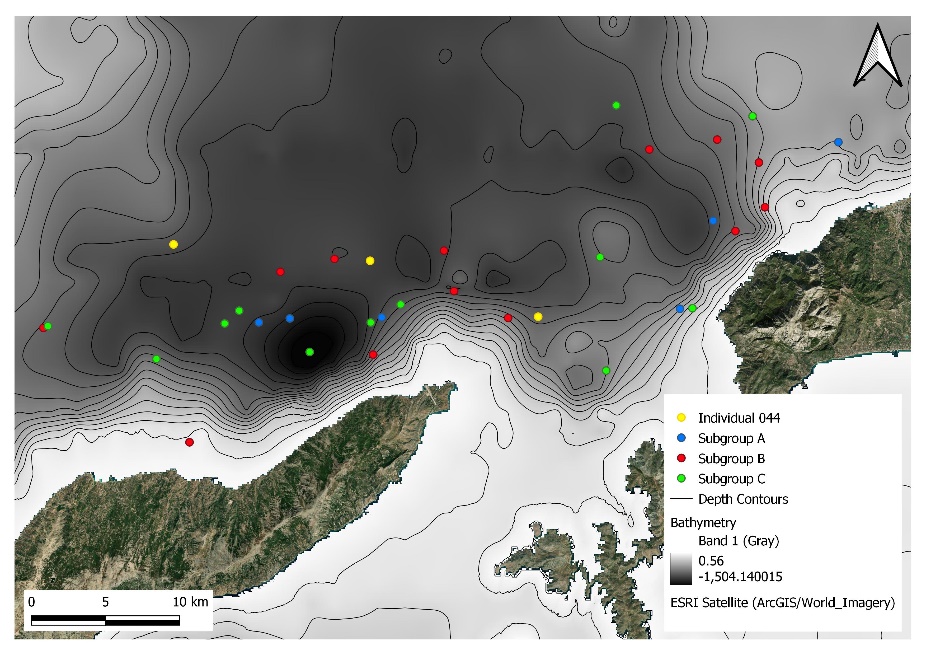
Figure 9. A map showing the distribution of Stenella coeruleoalba subgroups sighted in the trench of the eastern Aegean Sea north-west of Samos Island and north of Ikaria Island. Map created using QGIS.
Subgroup Depth
The results support the hypothesis that S. coeruleoalba subgroups cannot be defined by different depth ranges. Firstly, a Shapiro-Wilk test was performed to test for the normality of the depth values between the subgroups. The data was found to be non-significant, indicating normality (Shapiro-Wilk: p = 0.5701). Then, a one-way ANOVA was used to test for a significant difference between the mean depths at which the subgroups were observed. The result showed that the there was no significant difference in average depth between the different subgroups (one-way ANOVA: F3,35 = 0.89; p = 0.458). This suggests that the distribution of S. coeruleoalba subgroups in the eastern Aegean Sea are not determined by differences in depth preferences (Figure 10).
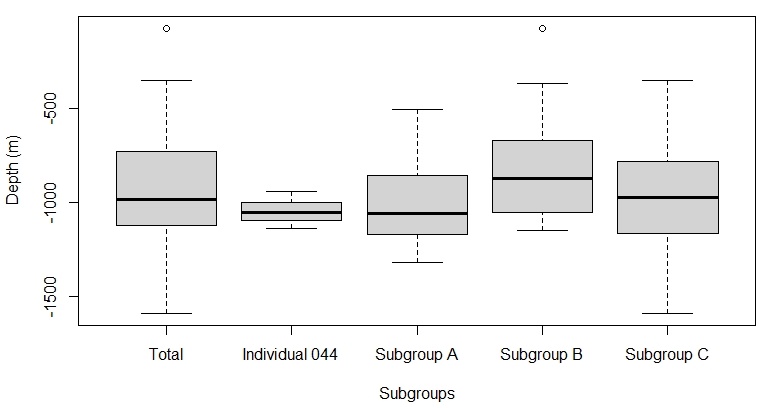
Figure 10. Box plots comparing the average depths (m) of Stenella coeruleoalba subgroup sightings. Tukey method box and whisker plots are displayed, where the thick black lines are the median values for each site. The average depth for the total population was 1000 metres. Plot created using RStudio.
Subgroup Distance from Shore
The findings from this study support the hypothesis that S. coeruleoalba subgroups cannot be defined by distance from shore. The distance values between the subgroups of the whole sub-population were tested for normality and the results showed that the data was normally distributed (Shapiro-Wilk: p = 0.615). There was no significant difference in distance from shore between the different subgroups (one-way ANOVA: F3,35 = 1.74; p = 0.177). The average distance from shore for the total population was 0.07 km. As there was no significant difference in distance from shore between the subgroups, this suggests that S. coeruleoalba subgroups in the eastern Aegean Sea cannot be defined or separated by differences in distance from shore preferences (Figure 11).
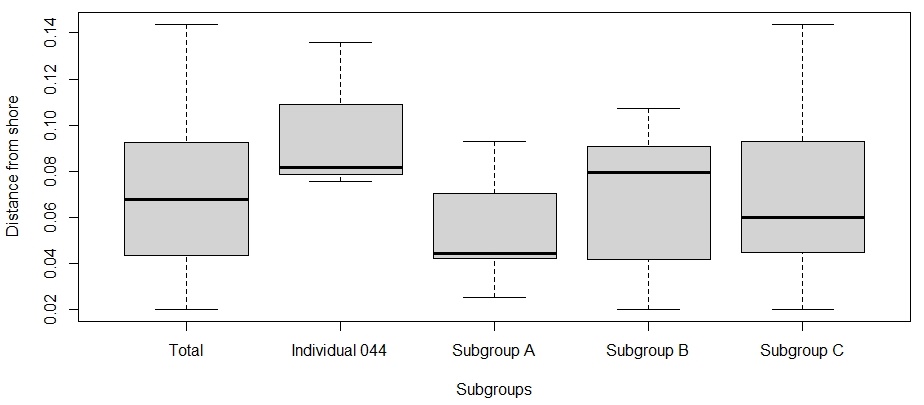
Figure 11. Box plots comparing the average distance from shore (km) of Stenella coeruleoalba subgroup sightings. Tukey method box and whisker plots are displayed, where the thick black lines are the median values for each site. Plot created using RStudio.
Discussion
The results from this study suggest that there is a presence of multiple S. coeruleoalba social subgroups in a subpopulation in the eastern Aegean Sea that largely differ in size. The data is supported by other studies such as NOAA Fisheries (2019), which reported that striped dolphin groups average between 25 and 100 individuals but have occasionally been seen in larger groups of up to several hundred and even thousands of animals (NOAA Fisheries, 2019). This current study was conducted on a small area in the Aegean Sea featuring one sub-population, therefore, it is highly likely that there are other subgroups of Stenella coeruleoalba further around the island or further into the Aegean Sea that should be included in further study. In addition, if more individuals could be identified, the subgroups may look different to those found in this study. The total number of individuals in this study was 27 and the largest subgroup consisted of 12 individuals. This is not an accurate account of the whole subpopulation of S. coeruleoabla in the eastern Aegean Sea as some individuals could not be identified due to their lack of identifiable features on their dorsal fins. Individual 044 had no social association with any of the other individuals included in this study. However, some individuals could not be identified, which had social associations with Individual 044. Smolker et al. (1992) found that males formed subgroups of two or three individuals who consistently associated with each other (Smolker et al., 1992). This could be the case for Individual 044, who may be in a separate subgroup with other unidentified male individuals or for Individuals 066 and 067, who were always sighted together. Sex was not used as an explanatory variable as it was difficult to identify the sex of each individual.
The results from the analyses of fluid social subgroups are similar to those reported by Ostman (1994) and by Blasi and Boitani (2014), who described that the social organisation of dolphins is highly fluid and that dolphins live in fission-fusion societies, where large schools form, break up and re-form daily (Ostman, 1994; Blasi and Bioitani, 2014). This study does not support the hypothesis that S. coeruleoalba subgroups can be defined by geographic location, depth, and distance from shore. Although these environmental factors do not have an observed effect on the social structure of the subpopulation studied, it has been concluded by Blasi and Boitani (2014) that striped dolphins' social organisation depends on a combination of socio-ecological, demographic, and anthropogenic factors including habitat structure (depth and distance from shore), predation risk, prey distribution, cultural transmission, male competition, breeding success, and risk of infanticide (Blasi and Boitani, 2014). In addition, multiple studies have concluded that subgroups of striped dolphins may be organized by developmental class, sex, breeding status, and the presence of offspring (Brunnick, 2000; NOAA Fisheries, 2019).
To conclude, this study has increased our understanding of the social structure of S. coeruleoalba in the eastern Aegean Sea. Analysing the social structure of S. coeruleoalba is important as complex social structure is a prominent feature in cetaceans for communication, reproduction, and protection, and can lead to behavioural diversity among and within a population. The results have given insight into how these species survive in groups and can help inform effective conservation actions, such as the best placement of marine protected areas (MPAs) around Samos and surrounding Greek islands. The social structure of resident dolphin populations in the Aegean Sea is still poorly understood. However, this study has increased our understanding of social structure concerning the Stenella coeruleoalba species and could stand as a preliminary study for future research into factors affecting the social structure of these dolphins. Social structure has been seen to change by mating season (Blasi and Boitani, 2014). This could be tested in future by comparing sightings of individuals during different seasons. In addition, to further this study, this subpopulation could be investigated to see if subgroups are organised by age or sex. This will need a more in-depth photo-identification catalogue, which considers and records these factors. It was stated by Johnson et al. (1986), that reliably determining the age and sex of wild dolphins may require the actual capture or performing biopsies on the animals involved (Johnson et al., 1986). However, underwater cameras can be used to photograph genitalia and analyse colour changes of individuals over time to help identify the sex of individuals in a non-invasive way.
References
Archer, F.I. (2018) ‘Striped Dolphin’, Encyclopedia of Marine Mammals, pp.954–956. Available at: https://doi.org/10.1016/b978-0-12-804327-1.00251-x
Barendse, J., Best, P.B., Carvalho, I. and Pomilla, C. (2013) ‘Mother Knows Best: Occurrence and Associations of Resighted Humpback Whales Suggest Maternally Derived Fidelity to a Southern Hemisphere Coastal Feeding Ground’, PLoS ONE, 8(12), p.e81238. Available at: https://doi.org/10.1371/journal.pone.0081238
Blasi, M.F. and Boitani, L. (2014) ‘Complex Social Structure of an Endangered Population of Bottlenose Dolphins (Tursiops truncatus) in the Aeolian Archipelago (Italy)’, PLoS ONE, 9(12), p.e114849. Available at: https://doi.org/10.1371/journal.pone.0114849
Brown, B. and Tintore, B. (2021) ‘Photo-Identification of Marine Mammals’, Archipelagos Institute of Marine Conservation. (Figures 3-6).
Brunnick, B.J. (2000) ‘The social organization of the Atlantic spotted dolphin’, Stenella frontalis, in the Bahamas – ProQuest’, UMI [online] Available at: https://www.proquest.com/openview/14f859d660d171d5b304ee8df9ee4cd4/1?pq-origsite=gscholar&cbl=18750&diss=y (Accessed 7 Feb. 2023).
Carlucci, R., Bas, A.A., Maglietta, R., Renò, V., Fanizza, C., Rizzo, A., Crugliano, R. and Cipriano, C. (2018) ‘Site fidelity, residency and habitat use of the Risso’s dolphin Grampus griseus in the Gulf of Taranto (Northern Ionian Sea, Central-eastern Mediterranean Sea) by photo-identification’, IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), pp. 173-177. Available at: https://doi.org/10.1109/MetroSea.2018.8657847
Cozzi, B., Huggenberger, S. and Oelschläger, H. (2017) ‘Natural History and Evolution of Dolphins: Short History of Dolphin Anatomy’, Anatomy of Dolphins, pp.1–20. Available at: https://doi.org/10.1016/b978-0-12-407229-9.00001-4
Elliser, C.R. and Herzing, D.L. (2013) ‘Long-term social structure of a resident community of Atlantic spotted dolphins, Stenella frontalis, in the Bahamas 1991-2002’, Marine Mammal Science, 30(1), pp.308–328. Available at: https://doi.org/10.1111/mms.12039
Foley, A., McGrath, D., Berrow, S. and Gerritsen, H. (2010) ‘Social Structure Within the Bottlenose Dolphin (Tursiops truncatus) Population in the Shannon Estuary, Ireland’, Aquatic Mammals, 36(4), pp.372–381. Available at: https://doi.org/10.1578/am.36.4.2010.372
Gaspari, S., Azzellino, A., Airoldi, S. and Hoelzel, A.R. (2007) ‘Social kin associations and genetic structuring of striped dolphin populations (Stenella coeruleoalba) in the Mediterranean Sea’, Molecular Ecology, 16(14), pp.2922–2933. Available at: https://doi.org/10.1111/j.1365-294x.2007.03295.x
Hupman, K., Stockin, K.A., Pollock, K., Pawley, M.D.M., Dwyer, S.L., Lea, C. and Tezanos-Pinto, G. (2018) ‘Correction: Challenges of implementing Mark-recapture studies on poorly marked gregarious delphinids’, PLOS ONE, 13(8), p.e0203356. Available at: https://doi.org/10.1371/journal.pone.0203356
Johnson, C.M., Norris, K.S., Schusterman, R.J., Thomas, Jeanette, A. and Wood, F.G. (1986) ‘Dolphin Cognition and Behavior: A Comparative Approach’, Google Books, Lawrence Erlbaum Associates Inc., pp.335–346. Available at: https://doi.org/10.4324/9780203767689
Mesnick, S.L., Ballance, L.T., Wade, P.R., Pryor, K. and Reeves, R.R. (2019) ‘Oceanic Dolphin Societies: Diversity, Complexity, and Conservation’, Ethology and Behavioral Ecology of Odontocetes, Ethology and Behavioral Ecology of Marine Mammals. Springer, Cham, pp 183–209. Available at: https://doi.org/10.1007/978-3-030-16663-2_9
NOAA Fisheries (2019) ‘Striped Dolphin’ , [online] National Oceanic and Atmospheric Administration. Available at: https://www.fisheries.noaa.gov/species/striped-dolphin (Accessed 6 Feb. 2023).
Ostman, J.S.O. (1994) ‘Social organization and social behavior of Hawai’ian spinner dolphins (Stenella longirostris)’, University of California, Santa Cruz ProQuest Dissertations Publishing. Available at: https://login.uniessexlib.idm.oclc.org/login?url=https://www.proquest.com/dissertations-theses/social-organization-behavior-hawaiian-spinner/docview/304110710/se-2?accountid=10766 (Accessed 14 Oct. 2024)
RStudio Team (2020) ‘RStudio: Integrated Development for R’, RStudio, PBC, Boston, MA. Available at: http://www.rstudio.com (Accessed 7 Feb. 2023).
Shapiro, S.S. and Wilk, M.B. (1965) ‘An analysis of variance test for normality (complete samples)’, Biometrika, 52(3–4), pp.591–611. Available at: https://doi.org/10.1093/biomet/52.3-4.591
Smolker, R.A., Richards, A.F., Connor, R.C. and Pepper, J.W. (1992) ‘Sex Differences in Patterns of Association Among Indian Ocean Bottlenose Dolphins’, Behaviour, 123(1-2), pp.38–69. Available at: https://doi.org/10.1163/156853992x00101
Society for Marine Mammalogy (2022) ‘Stenella coeruleoalba’, [online] marinemammalscience.org. Available at: https://marinemammalscience.org/facts/stenella-coeruleoalba (Accessed 28 Nov. 2022).
Tethys Research Institute (2020) ‘Striped dolphins: research-striped-dolphins-2’ [Online image] Tethys.org. Available at: https://tethys.org/activities-overview/research/striped-dolphins (Accessed 16 May 2023). (Figure 1).
Whitehead, H. (2009) ‘SOCPROG programs: analyzing animal social structures’, Behavioral Ecology and Sociobiology. Available at: https://doi.org/63:765-778
World Atlas (2021) ‘Aegean Sea’, [online] WorldAtlas. Available at: https://www.worldatlas.com/seas/aegean-sea.html (Accessed 28 Nov. 2022).
Copyright Statement
©Danielle Roth. This article is licensed under a Creative Commons Attribution 4.0 International Licence (CC BY 4.0).
